In the news about 3D printing as it relates to the field of medicine, from 3D printed pills to prosthetics, it might not occur that the committees that oversee and approve such applications for human use might dabble in 3D printing themselves. Of course, I’m referring to the US’s own Food and Drug Administration (FDA), the gatekeeper that decides the medications and technologies that make it to market.
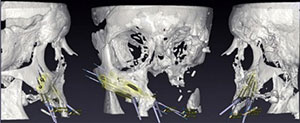
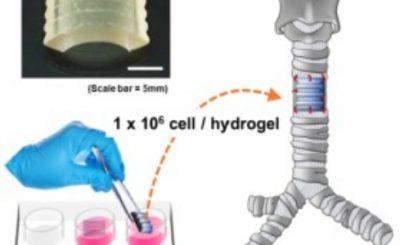
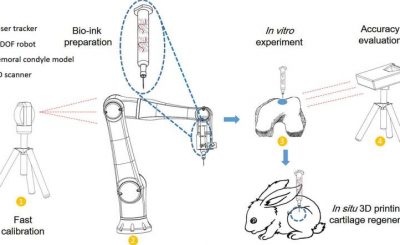
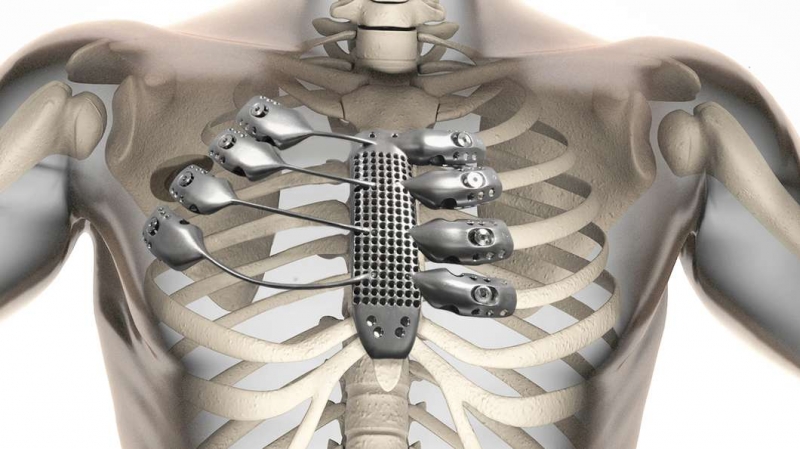
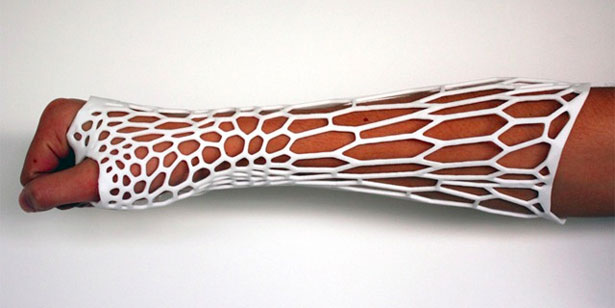
Aside from giving 3D-printed medical devices their stamp of approval – such as Oxford Performance Materials’  As 3D printing makes it possible to develop highly individualized forms of medical treatment, such as prosthetics specifically tailored to a given person, it becomes essential that personalized medical devices stand up to regulatory scrutiny. At the Functional Performance and Device Use Laboratory, researchers can tweak and 3D print new or modified designs for medical devices to determine if they fit and function properly and are safe for human use. The Laboratory for Solid Mechanics is investing the printing processes themselves, understanding which materials and forms of printing will achieve what sort of strength and durability in order to establish standards for AM used in the production of medical goods.
As 3D printing makes it possible to develop highly individualized forms of medical treatment, such as prosthetics specifically tailored to a given person, it becomes essential that personalized medical devices stand up to regulatory scrutiny. At the Functional Performance and Device Use Laboratory, researchers can tweak and 3D print new or modified designs for medical devices to determine if they fit and function properly and are safe for human use. The Laboratory for Solid Mechanics is investing the printing processes themselves, understanding which materials and forms of printing will achieve what sort of strength and durability in order to establish standards for AM used in the production of medical goods.

Now that additive manufacturing has made its way from cutting edge design firms to bureaucratic agencies, there’s no doubt that the technology is quickly becoming ubiquitous. And, because the medical industry is one of the areas that will be most rapidly transformed by 3D printing, it may be difficult to navigate the vast changes and investment opportunities brought about. Hopefully, 3DPI’s first industry report, which covers additive manufacturing as it relates to developments in healthcare, will be a source of comfort to you in this exhilarating – and, at times, confusing – world of 3D printing.
Source: FDA Voice